At Polpharma Group, all our efforts are focused on delivering high-quality and affordable medicines to as many communities as possible.
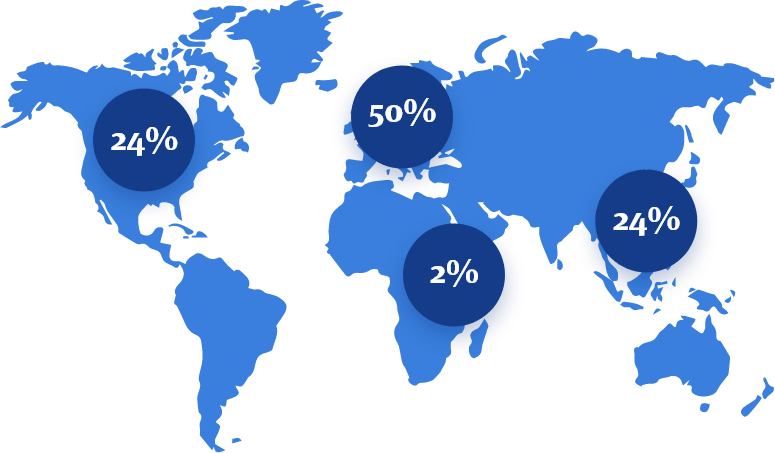
For over 80 years, trusted by patients, healthcare professionals, and business partners alike. A leading regional (CEE and CIS) manufacturer of pharmaceuticals and a leader in the Polish & Kazakhstan pharmaceutical market. Actively operating in Central and Eastern Europe, the Caucasus, and Central Asia markets.
Every day we diligently cooperate with doctors, pharmacists, and other healthcare professionals to better understand every stage of a patient’s journey and ensure that our patients receive and stick to the most adequate treatment.
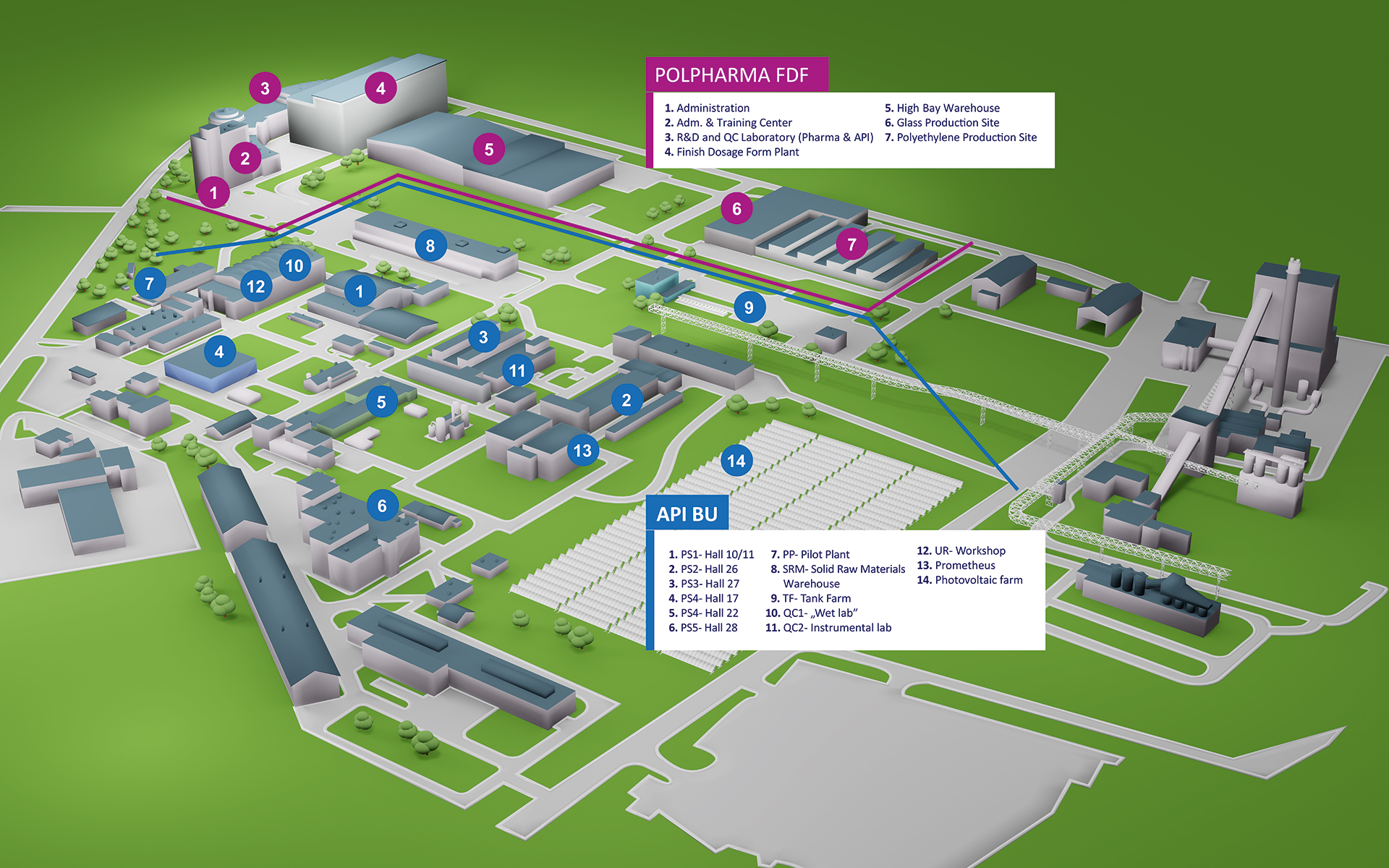
Thanks to best-in-class industry talents and state-of-the-art technologies, we are able to design and deliver crucial products and solutions to address the biggest health challenges (an aging population, chronic diseases) and healthcare system problems (increasing healthcare costs, resource shortages).
Through collaboration with our partners, we explore new ways of doing business and accelerate positive changes.
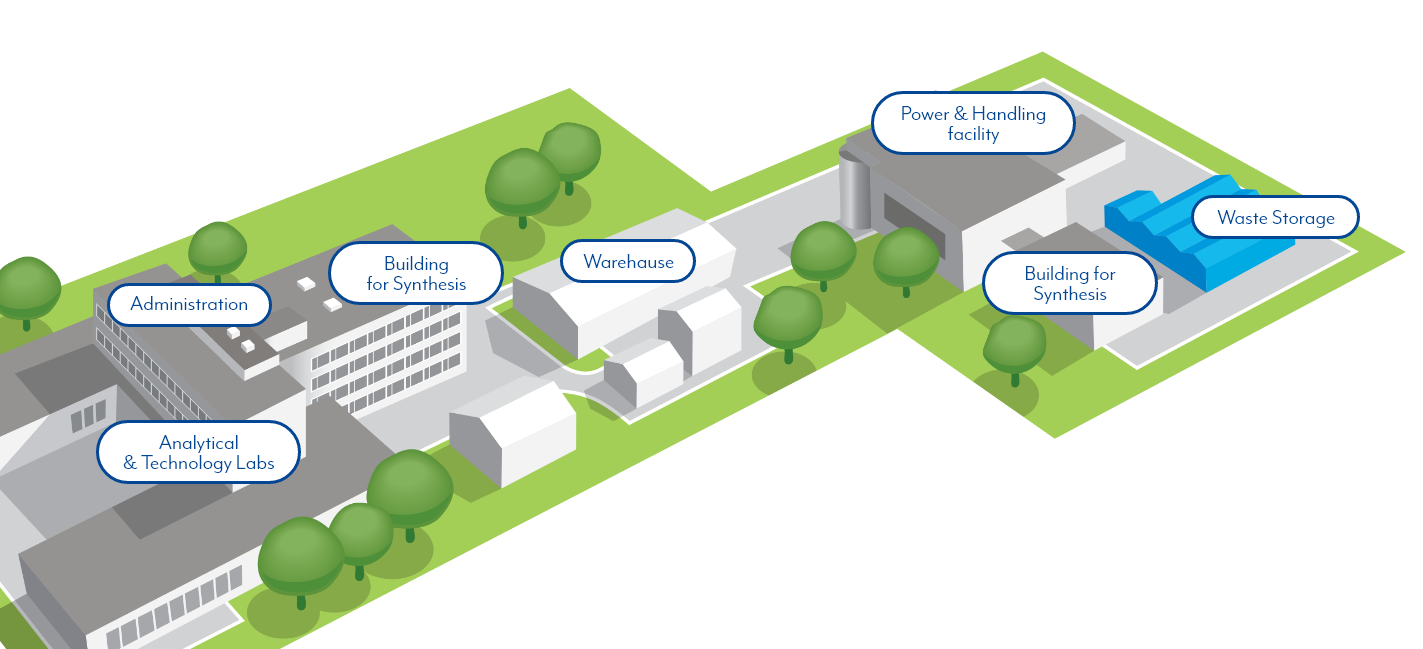
Polpharma Group Overview
- A leading regional (CEE and CIS) manufacturer of pharmaceuticals
-
Transforming the healthcare industry in terms of advanced commercial
models and the usage of AI to improve both the closeness to the HCP
professionals and patients and its effectiveness -
For over 85 years, trusted by patients, healthcare professionals,
and business partners alike.
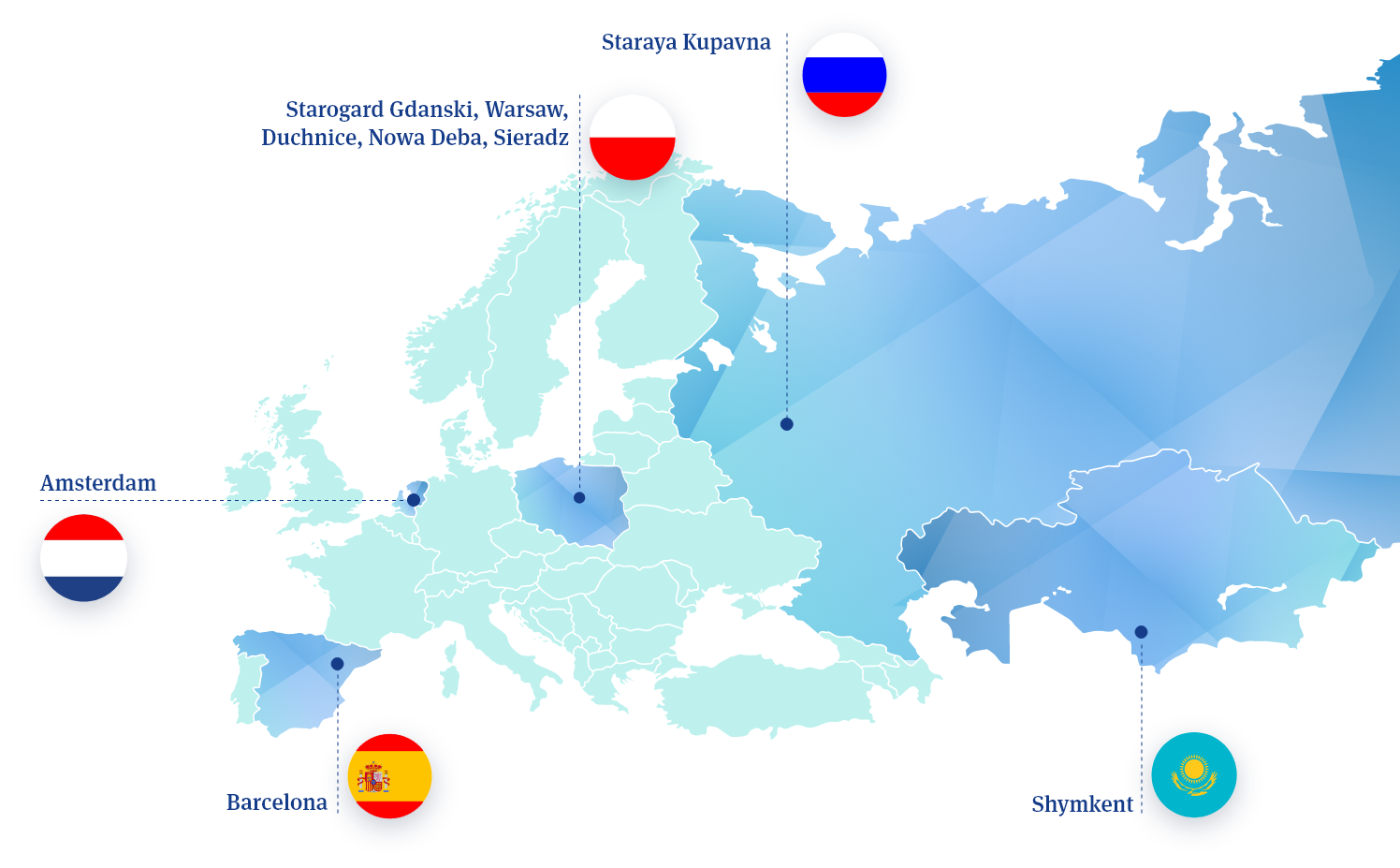
Key Facts:
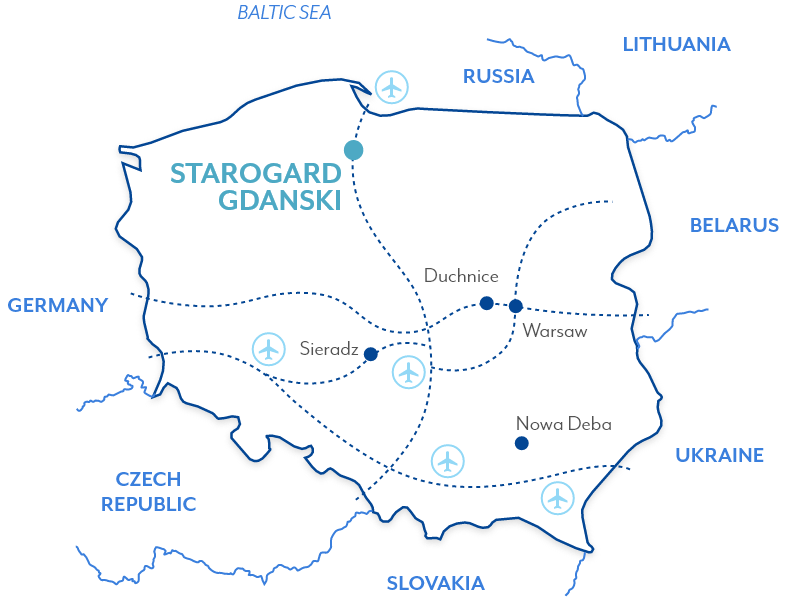
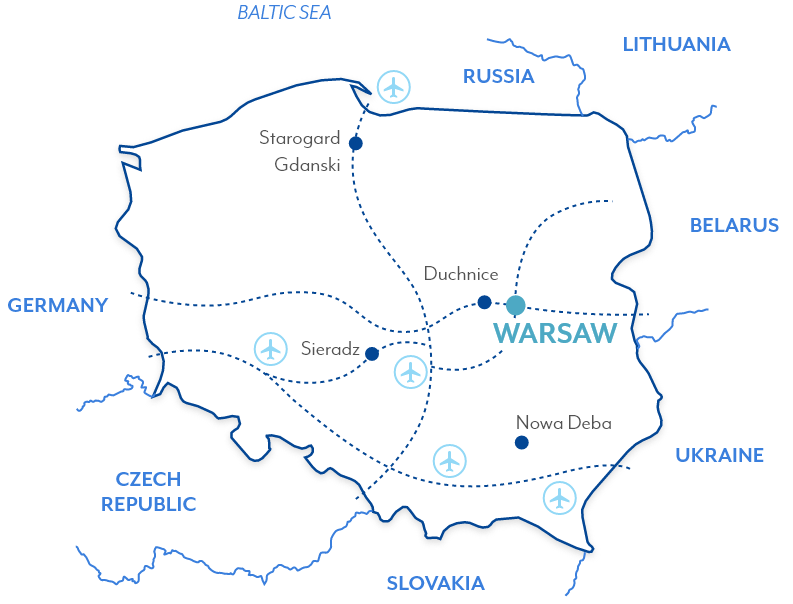
Starogard
Gdanski,
Shymkent, Staraya Kupavna
Russia & Kazachstan